Gas-phase reactions of cyclopropenylidene with protonated alkyl amines†
Abstract
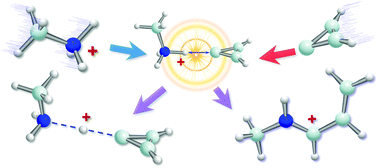
Vinylidene carbenes (C3H2) are of high interest to interstellar, combustion, and organic chemistry. Due to their high instability, the direct experimental investigation of their chemical reactivity has rarely been achieved. Herein, we report a first study on the reactions of cyclopropenylidene (c-C3H2) with protonated alkyl amines in the gas phase using a home-built ion trap mass spectrometer. The high gas-phase basicity (GB) of (1A1) c-C3H2 (calculated as 920 kJ mol−1) facilitates the formation of a proton-bound dimer with protonated amines as the first step in the reaction. The dimer can stay as it is or rearrange to a covalent product. The formation of the covalent complex is highly exothermic and its yield is affected by the GB of alkyl amines. The highest yield (82%) was achieved when the GB of the amine was slightly lower but comparable to that of c-C3H2. Our results demonstrate a new reaction pathway of c-C3H2, which has long been considered as a “dead end” in interstellar carbon chemistry.

 Please wait while we load your content...
Please wait while we load your content...