Porous Co–P foam as an efficient bifunctional electrocatalyst for hydrogen and oxygen evolution reactions†
Abstract
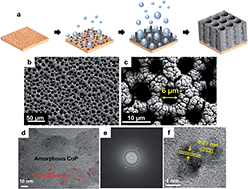
A high-performance bifunctional Co–P foam catalyst was successfully synthesized by facile one-step electrodeposition at a high cathodic current density. The synthetic approach includes fast generation of hydrogen bubbles as well as fast deposition of Co–P, which played a key role in forming a porous Co–P foam structure. The Co–P foam exhibits remarkable electrocatalytic activity and stability in both acidic and alkaline solution. Its HER activity was recorded with an overpotential of 50 mV in 0.5 M H2SO4 and 131 mV in 1 M KOH at 10 mA cm−2, which is comparable to that of commercial Pt/C (η@10 mA cm−20.5 M H2SO4: 33 mV, η10 mA, 1 M KOH: 80 mV). The Co–P foam (η@10 mA cm−2: 300 mV) exhibits better OER activities than Ir/C (η@10 mA cm−2: 345 mV) and RuO2 (η@10 mA cm−2: 359 mV) in 1 M KOH solution. The excellent performance of the Co–P foam as an HER and OER catalyst can be attributed to the charge separation between Co and P in Co–P foam as well as the porous foam structure providing a large electrochemically active surface area (ECSA). The ECSA of the Co–P foam was calculated to be 118 cm2, which was 2.4 times higher than that of a Co–P film (49 cm2).

 Please wait while we load your content...
Please wait while we load your content...