A one-step water based strategy for synthesizing hydrated vanadium pentoxide nanosheets from VO2(B) as free-standing electrodes for lithium battery applications†
Abstract
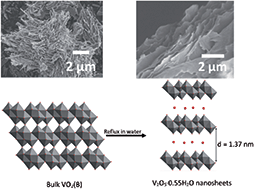
The synthesis of two dimensional (2D) materials from transition metal oxides, chalcogenides, and carbides mostly involve multiple exfoliation steps in which hazardous solvents and reagents are used. In this study, hydrated vanadium pentoxide (V2O5·nH2O) nanosheets with a thickness of a few nanometers were prepared via a facile environmentally friendly water based exfoliation technique. The exfoliation process involved refluxing the precursor, vanadium dioxide (VO2(B)), in water for a few days at 60 °C. The proposed exfoliation mechanism is based on the intercalation/insertion of water molecules into the VO2(B) crystals and the subsequent cleavage of the covalent bonds holding the layers of VO2(B) together. The thermal and chemical analyses showed that the approximate chemical composition of the nanosheets is H0.4V2O5·0.55H2O, and the percentage of VV content to that of VIV in the nanosheets is about 80(3)% to 20(3)%. The exfoliated aqueous suspension of the V2O5·0.55H2O nanosheets was successfully deposited onto multi-walled carbon nanotube (MW-CNT) paper to form free-standing electrodes with a thickness of the V2O5·0.55H2O layer ranging between 45 and 4 μm. A series of electrochemical tests were conducted on the electrodes to determine the cyclability and rate capability of lithium insertion into V2O5·0.55H2O nanosheets. The electrodes with the thinnest active material coating (∼4 μm) delivered gravimetric capacities of up to 480 and 280 mA h g−1 when cycled at current densities of 10 and 200 mA g−1, respectively.


 Please wait while we load your content...
Please wait while we load your content...