Controlling catalytic dehydrogenation of formic acid over low-cost transition metal-substituted AuPd nanoparticles immobilized by functionalized metal–organic frameworks at room temperature†
Abstract
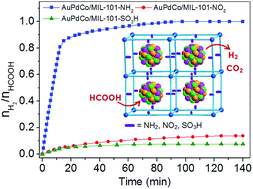
A series of nonprecious transition metal (Fe, Co and Ni)-substituted AuPd nanoparticles (NPs) immobilized by porous metal–organic frameworks (MOFs) containing different functional groups were synthesized, and their catalytic performance for the dehydrogenation of formic acid (HCOOH, FA) was studied. In comparison with AuPdCo NPs immobilized by bare MIL-101 and MIL-101 functionalized by electron-accepting groups NO2 and SO3H, which had very low H2 selectivity and activity, AuPdCo NPs immobilized by MIL-101 functionalized by electron-donating group NH2 exhibited 100% H2 selectivity and drastically high activity featuring a turnover frequency (TOF) value of 347 h−1 at 298 K, comparable to the reported values of highly active heterogeneous noble-metal catalysts and homogeneous catalysts. The remarkably different and controllable activity and H2 selectivity may be attributed to the different bonding modes between AuPdCo NPs and functionalized MOFs, which might lead to the different interaction pathways between the intermediates from the splitting of FA molecules and the metallic atoms on the catalyst surface.

 Please wait while we load your content...
Please wait while we load your content...