Tuning the CO oxidation catalytic activity of supported metal–metal oxide heterostructures by an aqueous phase post-treatment process†
Abstract
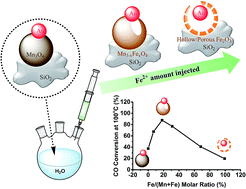
Colloidal Au–MnO heterodimers were deposited on SiO2 and calcined at high temperature in air in order to prepare a ligand-free Au–Mn3O4/SiO2 model catalyst for CO oxidation with a well-defined Au size and Au–metal oxide interface. The composition and morphology of the Mn3O4 nanosized domains within the dimers were then precisely and selectively modified by a simple post-treatment procedure, based on the reaction with Fe(ClO4)2 aqueous solutions. The post-treatment resulted in a significant improvement of the catalytic performance in the CO oxidation reaction. The sample which was characterized by a Fe/(Fe + Mn) molar ratio of ∼18% and which contained pinholes in the oxide domains exhibited the highest catalytic activity in the CO oxidation reaction. X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), energy dispersive X-ray spectroscopy (EDS), High Resolution Transmission Electron Microscopy (HRTEM), High-Angle Annular Dark Field (HAADF)-STEM and Temperature Programmed Reduction (TPR) were used to monitor the transformations induced by the post-treatment process on the Au–Mn3O4 nanocrystals (NCs). The replacement of Mn by Fe was accompanied by a progressive increase of the porosity of the oxide domains. By correlating the chemical and morphological evolution of the metal–metal oxide heterostructures with their catalytic performances, we found that the observed activity trend was mainly driven by the modification of the Au–metal oxide interface. The post-treatment process could be generalized to other metal oxide/metal cation pairs.


 Please wait while we load your content...
Please wait while we load your content...