In situ formation of NiO on Ni foam prepared with a novel leaven dough method as an outstanding electrocatalyst for oxygen evolution reactions†
Abstract
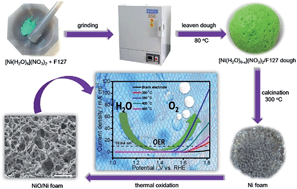
A novel leaven dough method was developed to prepare three-dimensional Ni foams, upon which a thin NiO nanostructure was grown through thermal oxidation to form NiO/Ni foams as a highly efficient electrocatalyst for oxygen evolution reactions (OERs). This method is simple, scalable, and carries less environmental burdens, involving no cumbersome time-consuming templating procedures and adopts a new concept of “solid solvent” instead of using a large amount of solvent. In this method, nickel(II) nitrate hexahydrate served as the Ni precursor and leavening agent, with F127 acting as a “solid solvent” and fuel. Heating the ground mixture of the nitrate and F127 powders at 80 °C led to formation of a green dough that was calcinated at 300 °C to obtain the Ni foam. The NiO/Ni foam was prepared by in situ thermal oxidation of the Ni foam and applied as an electrocatalyst for OERs. The NiO/Ni electrode fabricated from the NiO/Ni composite exhibited a remarkably low onset over-potential of 268 mV and low working over-potential of 345 mV at 10 mA cm−2. The electrochemical kinetics of the electrode were outstanding with an ultra-small Tafel slope of 53 mV dec−1 in 1 M KOH. The outstanding electrocatalytic performances of the present NiO/Ni electrode, comparable to those achieved by noble metal oxide based electrodes, can be attributed to the three-dimensionally well-connected Ni foam skeleton and the intact contact of the NiO/Ni domains, offering excellent charge transfer and transport networks. The present leaven dough method can be extended to fabricate a wide variety of metal oxide/metal foams for applications in electrochemical devices for intact functional material/current collector composite electrodes.

 Please wait while we load your content...
Please wait while we load your content...