Ethyl viologen dibromide as a novel dual redox shuttle for supercapacitors†
Abstract
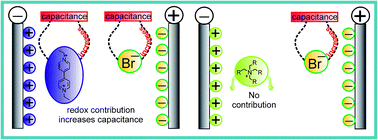
Viologen (1,1ʹ-diethyl-4,4ʹ-bipyridinium bromide) based redox active electrolyte in 1.0 M H2SO4 has been proposed as a novel electrolyte for supercapacitor (SC) applications due to its dual cathodic and anodic redox behaviour. Unlike other reported redox additives, viologen as a single redox species can improve the performance of both positive and negative electrodes simultaneously through the redox behaviour of bromide and 1,1ʹ-diethyl-4,4ʹ-bipyridinium ions. The synergic redox behaviour of the ions and their effect towards the enhancement of the electrochemical performance of the activated charcoal based SC are compared with those of neat H2SO4 and triethylbutylammonium bromide (N2224Br). The maximum specific capacitance of 408.0 F g−1 and specific energy of 23.0 W h kg−1 at 0.25 A g−1 were obtained for the viologen mediated SC. Interestingly, the specific capacitance continuously increased for the viologen mediated SC on charge–discharge cycling and 30% increment was observed at the end of 1000 cycles. The relaxation time constant is compared for SCs with viologen, neat H2SO4 and N2224Br electrolytes.

 Please wait while we load your content...
Please wait while we load your content...