Micro–mesoporous iron oxides with record efficiency for the decomposition of hydrogen peroxide: morphology driven catalysis for the degradation of organic contaminants†
Abstract
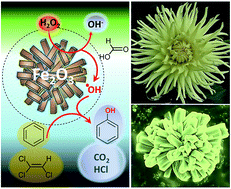
A template-free solid-state synthesis of a morphologically controlled and highly organized iron(III)oxide micro–mesoporous Fenton catalyst has been engineered through a simple two-step synthetic procedure. The 3D nanoassembly of hematite nanoparticles (5–7 nm) organized into a rod/flower-like morphology shows the highest rate constant reported to date for the decomposition of H2O2 (1.43 × 10−1 min−1) with superior efficiency for the degradation of aromatic (phenol, benzene, ethylbenzene) and chlorinated (trichloroethylene) pollutants in contaminated water. The morphological arrangement of nanoparticles is therefore considered one of the key variables that drive catalysis.

 Please wait while we load your content...
Please wait while we load your content...