Hydrous TiO2@polypyrrole hybrid nanocomposite as an efficient selective scavenger for the defluoridation of drinking water†
Abstract
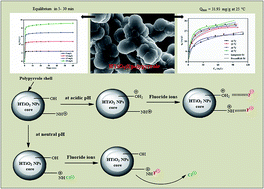
An adsorptive process for the defluoridation of drinking water was performed using a hybrid nanocomposite of hydrous titanium oxide@polypyrrole (HTiO2@PPy), as a scavenger. The adsorbent was successfully fabricated via facile in situ chemical oxidative polymerization of pyrrole monomer in aqueous media in which HTiO2 nanoparticles were suspended. The developed adsorbent was characterized using various spectro-analytical techniques viz. BET, FTIR, FE-SEM, STEM, EDX, TGA and ZETA SIZER. Relatively high BET surface area (98.17 m2 g−1) and pHpzc (∼8.4) values were obtained for HTiO2@PPy. The synergistic effect of both the counterparts (PPy and HTiO2) of the nanocomposite rapidly enhanced the F− adsorption process. A noteworthy rapid fluoride uptake best described by the pseudo-second-order kinetic model was observed (equilibrium attainment within 5–30 min). The Langmuir model best described the isotherm data with a maximum adsorption capacity of 31.93 mg g−1 at 25 °C and pH 6.5 (±0.2). Thermodynamic and activation parameters provided evidence of the spontaneous, endothermic and physical nature of the adsorption process. The selectivity of HTiO2@PPy for F− sorption was significant in the presence of Cl−, NO3−, HCO3−, SO42− and PO43− co-existing ions and noteworthy reusability for up to three regeneration cycles was achieved. Electrostatic interactions and ion-exchange were proposed to be the possible underlying mechanisms for the adsorption of F− by HTiO2@PPy nanocomposite. Thus, HTiO2@PPy is anticipated to serve as an efficient scavenger for the defluoridation of drinking water.

 Please wait while we load your content...
Please wait while we load your content...