Nitrogen-rich hypergolic ionic salts based on (2-methyltetrazol-5-yl)diazotates†
Abstract
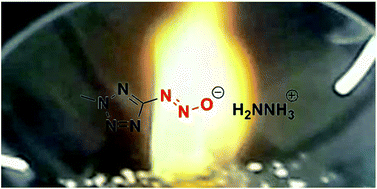
A series of hypergolic (2-methyltetrazol-5-yl)diazotates have been synthesized by treatment of bases with the diazotatic acid, which were prepared by using isoamyl nitrite at low temperature. These hypergolic energetic salts showed remarkable short ignition delay times (IDs) by droplet test and a high specific impulse up to 291.2 s by calculation. All compounds were fully characterized using IR spectroscopy, 1H and 13C NMR spectroscopy and differential scanning calorimetry (DSC), and, in the case of aminoguanidinium (2-methyltetrazol-5-yl)diazotate (8) and diaminoguanidinium (2-methyltetrazol-5-yl)diazotate (9), with single crystal X-ray structuring.

 Please wait while we load your content...
Please wait while we load your content...