Identification of 2-subsituted benzothiazole derivatives as triple-functional agents with potential for AD therapy†
Abstract
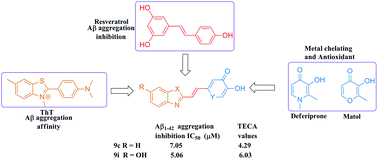
A novel series of 2-subsituted benzothiazole derivatives as MTDLs were designed and synthesized for AD therapy using pharmacophore-combine strategy. The benzothiazole moiety from ThT and the HPO moiety from deferiprone were connected with vinyl linker to achieve target compounds. The biological evaluation results revealed that the majority of them demonstrated desirable triple functions by interfering with Aβ aggregation, oxidative stress and metal dyshomeostasis simultaneously. The two most attractive compounds 9c and 9i exhibited excellent self-Aβ1–42 aggregation inhibitory activity, efficient ABTS˙+ scavenging activity, potent biometals chelating properties, as well as disaggregation activity against previous formed Aβ1–42 fibrils. In addition to these advantages, both of them displayed no cytotoxicity to human glioma U251 cells up to 50 μM, thereby meriting further investigation.

 Please wait while we load your content...
Please wait while we load your content...