Ni-based catalyst derived from Ni/Al hydrotalcite-like compounds by the urea hydrolysis method for CO methanation
Abstract
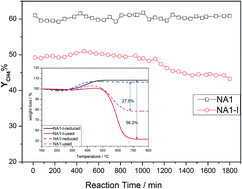
The catalytic methanation of CO was investigated at atmospheric pressure over Ni-based catalysts derived from Ni/Al hydrotalcite-like compounds. The catalysts were prepared by the urea hydrolysis method and characterized by using XRD, TGA, N2 adsorption–desorption, H2-TPR, H2-TPD and TEM. The H2-TPD analysis revealed an increase in the Ni surface area, from 14.9 to 21.8 m2 g−1, as the Ni/Al molar ratio was increased from 0.5 to 2, and a nearly constant Ni surface area, of about 22.3 m2 g−1, at Ni/Al molar ratios > 2. A similar dependence on Ni/Al molar ratio was also found for CO conversion. The NA1 (Ni/Al molar ratio to 1) catalyst exhibited excellent stability at 600 °C for 1800 min on the stream. The NA1 catalyst prepared from Ni/Al hydrotalcite-like compounds exhibited higher catalytic stability due to higher Ni dispersion and stronger resistance to coke deposition than did the impregnated NA1-I catalyst.

 Please wait while we load your content...
Please wait while we load your content...