Identification of bisindolylmethane–hydrazone hybrids as novel inhibitors of β-glucuronidase, DFT, and in silico SAR intimations†
Abstract
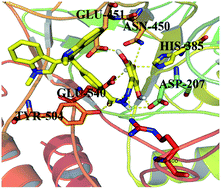
The present study involves the synthesis of bisindolylmethane–hydrazone hybrids, 1–30, in a three-step reaction sequence, followed by evaluation against β-glucuronidase enzyme. The IC50 values for the potent compounds were in the range from 0.10 to 83.50 μM. Compound, a trihydroxy analog was found to be the most potent derivative, having an IC50 value of 0.10 ± 0.001 μM. Molecular docking revealed that the active compounds could fit perfectly into the binding groove of β-D-glucuronidase. The presence of hydroxyl groups on the aromatic side chain proved to be the single most important factor that contributed toward the inhibitory potential of these compounds. On the other hand, the imino group of the hydrazone linkage displayed interactions with the side chain carboxyl oxygen (Oε2) of Asp207. The high inhibitory potential of these compounds could be associated with these strong hydrogen bonds. Structures of all the synthesized compounds were confirmed using modern spectroscopic methods.

 Please wait while we load your content...
Please wait while we load your content...