Precise grafting of macrocyclics and dendrons to a linear polymer chain†
Abstract
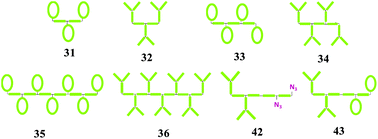
Sequential growth of multifunctional telechelic polymer chains was used here to produce three, four or seven HO-functionalities equally spaced along the polymer backbone. The telechelic building block consisted of a halide group on one end and both a hydroxyl and alkyne group on the other end. The key was to maintain the halide end-group on the telechelic polymer chain during the CuAAC coupling reaction after each sequential growth. This was accomplished by using the combination of the PMDETA ligand and toluene as a solvent to produce significantly faster rates of CuAAC coupling reaction over halide abstraction. The HO-functionalities were then converted to azide groups allowing further CuAAC reactions with either alkyne polymeric dendrons or cyclics to produce equally spaced grafts along the backbone. The relative hydrodynamic volume ratios of these grafted structures to their corresponding linear analogues were lower but similar to that found from the graft (i.e. dendron or cyclic) itself. Moreover, the same relative change in the hydrodynamic volume was independent of the number of grafts, suggesting that by combining many dendrons or cyclics on a polymer backbone, the effect on the coil conformation was minimal. Our methodology could be extended to produce an asymmetric block copolymer consisting of one block with two dendron grafts and the other block with two cyclic grafts. The work presented here will further extend the utility of both ‘living’ radical polymerization and ‘click’ reactions to produce complex polymer architectures.

 Please wait while we load your content...
Please wait while we load your content...