The application of double click to synthesize a third-order nonlinear polymer containing donor–acceptor chromophores†
Abstract
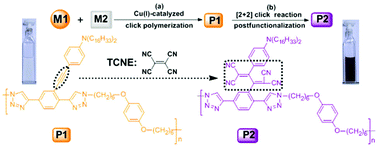
A new linear polymer containing the dialkylaniline-substituted electron-rich alkynes in the side chains was designed and synthesized by copper-catalyzed azide–alkyne cycloaddition (CuAAC). Subsequently, donor–acceptor chromophores were introduced into the side chains of the polymer by the [2 + 2] click reaction as an efficient postfunctionalization method. The polymers showed good solubility in common organic solvents and a high thermal stability. The photophysical and electrochemical properties, as well as the click reactions, were characterized by means of UV-vis absorption spectroscopy and cyclic voltammetry. After the introduction of donor–acceptor chromophores, the polymer showed a strong charge-transfer (CT) band in the visible absorption region, potent redox activities and a narrow band gap (1.36 eV). In addition, the third-order nonlinear properties, including the nonlinear absorption and the nonlinear susceptibilities, were investigated by using the Z-scan technique. A typical saturable absorption behavior was observed for the third order nonlinear absorption, with the nonlinear absorption coefficient (β) values of the polymer being −5.8 × 10−12 m W−1.

 Please wait while we load your content...
Please wait while we load your content...