Protonated montmorillonite-mediated highly specific isomerization of oleanolic acid esters: application to the synthesis of Δ13(18)-CDDO-Me†
Abstract
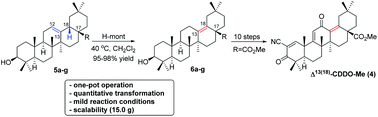
A one-pot highly specific isomerization of oleanolic acid esters (5a–g) to δ-oleanolic acid esters (6a–g) was achieved in the presence of proton-exchanged montmorillonite (H-mont) under mild reaction conditions. This protocol could proceed smoothly on a 15.0 gram scale. Based on this methodology, the synthesis of the biologically active compound Δ13(18)-CDDO-Me (4) was realized.

 Please wait while we load your content...
Please wait while we load your content...