A catalyst- and solvent-free thermal multicomponent approach for the construction of diverse and polysubstituted 2-aminopyridines and their antibacterial activity†
Abstract
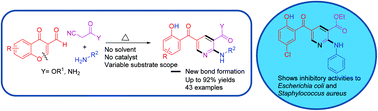
A highly efficient and facile thermal multicomponent cascade reaction of readily available 4-oxo-4H-chromene-3-carbaldehydes with cyanoacetates or cyanoamides and anilines or aliphatic amines under solvent and catalyst-free conditions was developed for the synthesis of a range of polysubstituted 2-aminopyridine derivatives in good to excellent yield. This protocol proceeds via a cascade reaction of Knoevenagel condensation, nucleophilic addition, intramolecular Michael addition, and ring opening. As an application of this methodology, the antibacterial activities of some of the synthetic compounds were evaluated.


 Please wait while we load your content...
Please wait while we load your content...