Hypervalent iodine(iii)-promoted N-incorporation into N-aryl vinylogous carbamates to quinoxaline diesters: access to 1,4,5,8-tetraazaphenanthrene†
Abstract
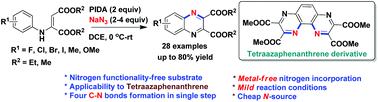
A novel oxidative N-incorporation strategy for synthesis of quinoxaline diesters under metal-free conditions is described for the first time. The mild reaction conditions allow for this transformation via the formation of two C(sp2)–N bonds utilizing cheaply available NaN3 as the N-atom source. N-Aryl vinylogous carbamates in this study undergo azidation at enamino C(sp2)–H selectively. The robustness of this strategy is further demonstrated by the synthesis of a valuable 1,4,5,8-tetraazaphenanthrene derivative using a mild and convenient approach.


 Please wait while we load your content...
Please wait while we load your content...