Addition of 4-(cyclohex-1-en-1-yl)morpholine on 3-nitroindole: an unprecedented dearomatizing process†
Abstract
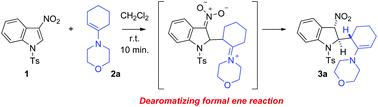
Nucleophilic enamines add spontaneously on heteroarenes 3-nitroindole and benzofuran to form a new C–C bond. With nitroindole 1 and enamine 2a, an unprecedented dearomatizing formal ene reaction occurs in a totally regio- and diastereo-selective manner. With 3-nitrobenzofuran 10 and enamine 2d, the reaction course is different and leads to the generation of a dienylphenol.


 Please wait while we load your content...
Please wait while we load your content...