Large-scale aerosol-assisted synthesis of biofriendly Fe2O3 yolk–shell particles: a promising support for enzyme immobilization†
Abstract
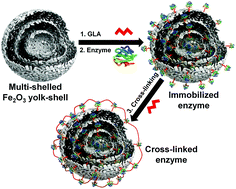
Multiple-shelled Fe2O3 yolk–shell particles were synthesized using the spray drying method and intended as a suitable support for the immobilization of commercial enzymes such as glucose oxidase (GOx), horseradish peroxidase (HRP), and laccase as model enzymes. Yolk–shell particles have an average diameter of 1–3 μm with pore diameters in the range of 16 to 28 nm. The maximum immobilization of GOx, HRP, and laccase resulted in the enzyme loading of 292, 307 and 398 mg per g of support, respectively. After cross-linking of immobilized laccase by glutaraldehyde, immobilization efficiency was improved from 83.5% to 90.2%. Km and Vmax values were 41.5 μM and 1722 μmol min−1 per mg protein for cross-linked laccase and those for free laccase were 29.3 μM and 1890 μmol min−1 per mg protein, respectively. The thermal stability of the enzyme was enhanced up to 18-fold upon cross-linking, and the enzyme retained 93.1% of residual activity after ten cycles of reuse. The immobilized enzyme has shown up to 32-fold higher stability than the free enzyme towards different solvents and it showed higher efficiency than free laccase in the decolorization of dyes and degradation of bisphenol A. The synthesized yolk–shell particles have 3-fold higher enzyme loading efficiency and lower acute toxicity than the commercial Fe2O3 spherical particles. Therefore, the use of unique yolk–shell structure Fe2O3 particles with multiple-shells will be promising for the immobilization of various enzymes in biotechnological applications with improved electrochemical properties. To the best of our knowledge, this is the first report on the use of one pot synthesized Fe2O3 yolk–shell structure particles for the immobilization of enzymes.

 Please wait while we load your content...
Please wait while we load your content...