Phosphorus–nitrogen compounds. Part 36. Syntheses, Langmuir–Blodgett thin films and biological activities of spiro-bino-spiro trimeric phosphazenes†
Abstract
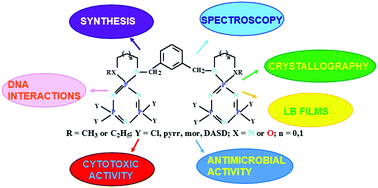
The condensation reactions of hexachlorocyclotriphosphazene (N3P3Cl6, trimer) with the symmetric N2N2 or N2O2 donor type tetradentate bulky ligands (1–4) gave partly substituted spiro-bino-spiro (sbs) (5–8) trimeric phosphazenes. Compounds 5–8 reacted with pyrrolidine, morpholine and 1,4-dioxa-8-azaspiro[4,5]decane (DASD) to give octapyrrolidino- (9–12), morpholino- (13–16) and DASD-substituted cyclotriphosphazenes (17–20). The structures of the phosphazenes have been elucidated using FTIR, MS, 1H, 13C{1H} and 31P{1H} NMR, and HSQC spectral data. The molecular and solid-state structures of 5, 6 and 12 were verified by single crystal X-ray diffraction techniques. On the other hand, the ultrathin and highly ordered Langmuir–Blodgett (LB) films of compounds 6, 7, 9 and 12 were also fabricated. The structural characterization of the LB films was made using p-polarized grazing angle (GAIR) and horizontal attenuated total reflectance (HATR) techniques. All the novel phosphazene derivatives were evaluated for antibacterial activities against Gram-positive (G+) and Gram-negative (G−) bacteria and for antifungal activities against yeast strains. In addition, the cytotoxic effects of compounds 9, 13, 15, 16, 19 and 20 were investigated against L929 fibroblast and MDA-MB-231 breast cancer cells. The most active one among these compounds was compound 9 at 6.25 μg mL−1 concentration. The interactions between compounds 5–20 and pBR322 plasmid DNA were determined by agarose gel electrophoresis.

 Please wait while we load your content...
Please wait while we load your content...