An ion pair receptor facilitating the extraction of chloride salt from the aqueous to the organic phase†
Abstract
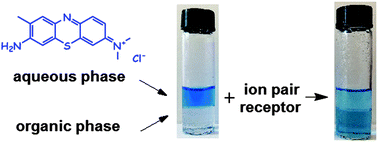
A new multitopic salt receptor based on an L-ornithine scaffold was synthesized using a simple approach; this receptor consists of a crown ether based cation and two squaramide anion binding domains. The binding properties of this receptor were measured spectrophotometrically, in acetonitrile solution. A comparative binding study of reference receptors enabled the evaluation of the role of particular binding domains in salt binding. The collected data revealed a stronger contribution of the anion binding domain located in the side chain of amino acid than the α-supported one. The cooperative action of these two binding domains is needed to strongly associate salts. Receptor 1 was found to be able to extract chloride salt from the aqueous to the organic phase.

 Please wait while we load your content...
Please wait while we load your content...