Salen-based enantiomeric polymers for enantioselective recognition†
Abstract
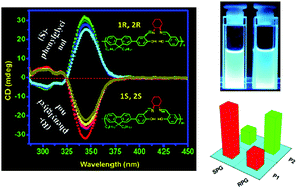
This paper describes how the spatial arrangement of the building blocks in a polymer chain influences the recognition properties of polymers. Here, we have designed and synthesized a series of fluorene based (A–B)n type salen polymers where the A-part is a fluorophore and the B-part is a recognition site using the condensation reaction of a fluorene based di(salicylaldehyde) compound (FSal) with diamines. Among the polymers, two are chiral (P1 and P2) which are enantiomeric (1R,2R vs. 1S,2S) to each other and three are achiral polymers (P3, P4 and P5). Circular dichroism studies of two chiral polymers reveal more or less equal and opposite CD intensities, indicating the induction of handedness in the polymer backbone. All polymers (P1–P5) contain the same recognition site but the spatial arrangements of the recognition sites are different from one another. We have studied the recognition properties of the polymers towards phenylglycinol. Only P1 and P2 exhibit good enantioselective recognition towards phenylglycinol through “turn-on” fluorescence enhancement because the microenvironment of the recognition sites is well organized to accommodate guest molecules. Three achiral polymers P3, P4 and P5 have no capacity to capture the guest molecules due to the lack of a suitable microenvironment at the recognition sites. This observation supports the phenomenon that the spatial arrangement of the building blocks in a main chain polymer determines its recognition properties. Moreover, P1 and P2 recognize the guest molecules through “turn-on” fluorescence (bright blue) in solution which gives a good platform for real application purposes.

 Please wait while we load your content...
Please wait while we load your content...