Synthesis, characterization and application of γ-MnO2/graphene oxide for the selective aerobic oxidation of benzyl alcohols to corresponding carbonyl compounds†
Abstract
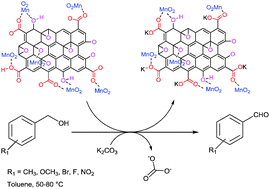
A facile low temperature approach was used to synthesize γ-MnO2 on the surface of graphene oxide (GO) through a simple wet precipitation method using MnSO4 as a precursor. X-ray diffraction analysis and Raman spectroscopy confirmed the formation of γ-phase MnO2 in the MnO2/GO nanocomposites. Transmission electron microscopy studies showed that γ-MnO2 exists as flower and needle structures in GO with an average size of approximately 15 nm. Inductively coupled plasma atomic emission spectroscopy studies confirmed a 62.5 wt% loading of γ-MnO2 in the GO nanocomposites. The γ-MnO2/GO catalyst shows good activity for the selective aerobic oxidation of benzyl alcohols to corresponding carbonyl compounds even when present in sub-stoichiometric amounts giving 91% yield over 3 h under mild reaction conditions. The catalyst showed high activity even after three cycles indicating good recyclability.


 Please wait while we load your content...
Please wait while we load your content...