EPR spectroscopy study of di-o-quinone bridged by π-extended TTF: redox behavior and binding modes as a ligand†
Abstract
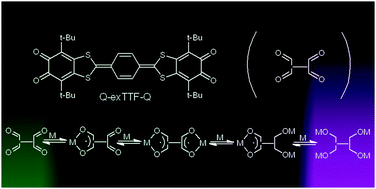
The peculiarities of the spin density distribution in the radical-anion form of an acceptor–donor–acceptor (A–D–A) system, consisting of two sterically hindered o-quinone moieties bridged by a p-phenylene extended tetrathiafulvalene molecule, was studied. It was chemically reduced with alkali metals. The reduction proceeds through four one-electron stages. The radical-anion and radical-trianion species are paramagnetic, whereas the dianion form was found to be EPR silent. The spin density distribution in the paramagnetic forms was studied. EPR and UV-vis spectra analyses and DFT calculations reveal that the p-phenylene extended TTF fragment in the ligand in all of the studied redox forms acts as a conducting bridge that provides electronic communication between the two o-quinone moieties.

 Please wait while we load your content...
Please wait while we load your content...