Transition metal phosphonates with supramolecular structures: syntheses, structures, surface photovoltage and luminescence properties†
Abstract
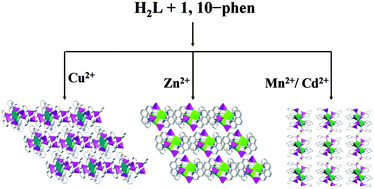
Four new transition metal phosphonates with 2D and 3D supramolecular structures, namely [Cu2(1,10-phen)2(HL)2(L)]·H2L (1), [Zn(1,10-phen)(HL)2] (2), [M(1,10-phen)(HL)2(H2L)] (M = Mn (3), and Cd (4)) (H2L = phenylphosphonic acid, 1,10-phen = 1,10-phenanthroline), have been synthesized under hydrothermal conditions. In compound 1, two {CuN2O3} square pyramids and three {CPO3} tetrahedra are interconnected through phosphonate oxygen atoms to form a cluster unit via edge- and corner-sharing. Neighboring units are further assembled into a 3D supramolecular structure through π–π stacking and hydrogen bonding interactions. For compound 2, two {ZnO3N2} square pyramids and four {CPO3} tetrahedra are interconnected by phosphonate oxygen atoms to a cluster unit via corner-sharing. Then the adjacent clusters are further assembled into a 3D supramolecular structure through π–π stacking interactions. Compounds 3 and 4 are isomorphous and adopt a 2D supramolecular structure. The interconnection of {MO4N2} and {CPO3} polyhedra leads to a cluster unit through corner-sharing, and such units are further assembled into a 2D supramolecular structure by π–π stacking and hydrogen bonding interactions. The surface photovoltage properties of compounds 1 and 3 and luminescence properties of compounds 2 and 4 have been studied. The surface photovoltage spectra (SPS) and field-induced surface photovoltage spectra (FISPS) of compounds 1 and 3 indicate that they all possess certain photo-electric conversion properties and show p-type semiconductor characteristics. Luminescence properties of compounds 2 and 4 indicate that they may be candidates for potential luminescent materials.

 Please wait while we load your content...
Please wait while we load your content...