Detailed characterization of lysozyme (Lyz)–surfactant (SDDS) interaction and the structural transitions†
Abstract
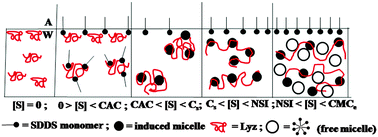
Surfactant interaction can influence the protein structure manifesting molecular unfolding–folding. The interaction can also affect the self-assembly formation of the surfactant. We studied the interaction of the protein lysozyme (Lyz) with the anionic surfactant sodium-N-dodecanoyl sarcosinate (SDDS) employing different methods viz., surface tension, viscosity, spectrophotometry, calorimetry and circular dichroism. Lyz structure was fairly perturbed by SDDS. Initially, SDDS monomers interacted with the protein, afterwards the formed small induced micelles of the surfactant became bound to the denatured Lyz, and finally the surfactant formed free micelles in solution that resided in the folds of the protein and in the bulk. These stage-wise interactions produced distinct transitions in the studied physicochemical properties with fair correlation with the altered protein structure found from circular dichroism (CD) measurements and the stepwise enthalpy values found from isothermal titration calorimetry (ITC). This was a unique corroboration of results. SDDS-denatured Lyz was reasonably renatured by β-cyclodextrin (β-CD) by stripping off the surfactant from the protein forming β-CD–SDDS adducts.

 Please wait while we load your content...
Please wait while we load your content...