Novel indole–flutimide heterocycles with activity against influenza PA endonuclease and hepatitis C virus†
Abstract
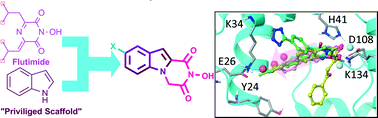
Influenza viruses cause considerable morbidity and mortality, whether in the context of annual epidemics, sporadic pandemics, or outbreaks of avian influenza virus. For hepatitis C virus (HCV), an estimated 170 million people are chronically infected worldwide. These individuals are at high risk of developing progressive liver injury or hepatocellular carcinoma. Since the efficacy of currently approved antiviral drugs is threatened by emerging viral resistance and the cost remains high, new antiviral drugs are still required. By utilizing a structure-based approach, novel substituted indole–flutimide heterocyclic derivatives (1,2-annulated indolediketopiperazines) were rationally designed, synthesized and evaluated as influenza PA endonuclease inhibitors. The compounds were also tested for their antiviral effect against HCV. All N-hydroxyimides were potent PA endonuclease inhibitors while displaying low cytotoxicity. Compound 6 proved to be the most active analogue, while the most favorable indole substitution was fluorine at position 8 (compound 18). The chloro-derivative 24 showed additional potent anti-HCV activity and exhibited remarkable selectivity (>19). In accordance with the SAR data, removal of the hydroxyl group from the imidic nitrogen (compound 26) caused a complete loss of activity against influenza PA endonuclease as well as HCV.

 Please wait while we load your content...
Please wait while we load your content...