Stabilizing high voltage LiCoO2 cathode in aqueous electrolyte with interphase-forming additive†
Abstract
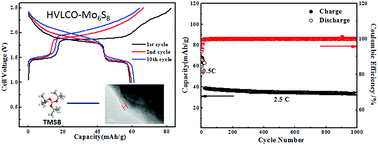
Aqueous lithium ion batteries (ALIBs) receive increasing attention due to their intrinsic non-flammable nature. Their practical application, however, has been prevented by the poor electrochemical stability of aqueous electrolytes, which severely restricts the choice of anode and cathode materials for such batteries and leads to low energy densities. It has been demonstrated that “water-in-salt” electrolytes can provide an expanded voltage window of ∼3.0 V, which can accommodate electrode materials that were otherwise forbidden in conventional aqueous electrolytes. In this study, we showed that the presence of an additive could further stabilize the aqueous electrolyte at a high voltage cathode surface. By forming a cathode electrolyte interphase (CEI) through electrochemical oxidation of tris(trimethylsilyl) borate (TMSB), we successfully stabilized LiCoO2 in water-in-salt electrolyte at a high cut-off voltage that corresponds to 0.7e electron charge transfer. To the best of our knowledge, this is also the first electrolyte additive known to form an interphase in aqueous electrolytes. The formed CEI significantly suppressed electrolyte oxidation as well as cobalt dissolution from LiCoO2 into electrolytes, allowing LiCoO2 to be stably charged/discharged at a higher cut-off voltage with a high capacity utilization of 170 mA h g−1. When paired with an Mo6S8 anode, the 2.5 V aqueous full cell delivered a high energy density of 120 W h kg−1 for 1000 cycles with an extremely low capacity decay rate of 0.013% per cycle.

 Please wait while we load your content...
Please wait while we load your content...