An ethylene glycol intercalated monometallic layered double hydroxide based on iron as an efficient bifunctional catalyst†
Abstract
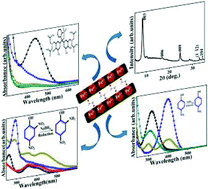
Given the fact that the literature describing the intercalation of organic molecules in monometallic LDH systems is scarce, the present investigation is aimed at the generation of ethylene glycol intercalated FeII–FeIII LDH with the objective of enhancing the surface area for further catalytic applications of industrially important and environmentally harmful organics. The solvothermal reaction of FeCl3 with urea in an ethylene glycol medium yielded a brown colored powder which was characterized employing a wide range of analytical techniques including high resolution powder X-ray diffraction (PXRD), scanning electron microscopy, thermal analysis, X-ray photo electron spectroscopy (XPS), elemental (C, H, N and S) analysis, UV-visible, photoluminescence spectroscopy measurements, BET surface area and pore-size analysis. The observed reflections in the PXRD pattern were indexed in a rhombohedral symmetry with a = 3.175 and c = 31.9 Å. Combining the results from the Fe 2p core level analysis and anion contents from elemental and thermogravimetric analysis, a formula of Fe2+1.06 Fe3+0.94 (O2C2H4) (OH)4 was deduced for the sample. The intercalation of EG in the interlayer was confirmed from FTIR and Raman spectroscopy measurements. The d–d transitions of the Fe3+-ion and the charge transfer transition of the Fe(II)–Fe(III) lattice were evident in the UV-visible spectrum. Blue indigoid emission bands arising from the transitions present in the Fe3+-ion were noticed in the photoluminescence spectrum. The measured BET surface area and pore diameter of the sample were 144 m2 g−1 and 12.5 nm, respectively. Almost instant decolourisation of the Xylenol Orange (XO) dye occurred in the presence of H2O2 and the LDH sample as catalyst. Similar observations were encountered for Methyl Orange (MO) and Methylene Blue (MB) dyes. All these reactions followed pseudo first-order kinetics. The industrially important reductive conversion of nitro aromatics was catalyzed by the sample. The selective reduction of 2,4-dinitrophenol to 2-amino-4-nitrophenol was effected almost instantaneously by this catalyst. Both the reusability and possible mechanism of catalytic action have been discussed. The EG intercalated Fe2+–Fe3+ LDH influenced the relaxivity value of protons as determined from NMR spectroscopy experiments.


 Please wait while we load your content...
Please wait while we load your content...