Syntheses, characterization and electrochemical and spectroscopic properties of ruthenium–iron complexes of 2,3,5,6-tetrakis(2-pyridyl)pyrazine and ferrocene-acetylide ligands†
Abstract
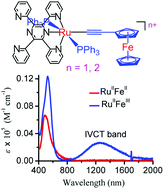
Heterodimetallic Ru–Fe complexes [(tppz)(PPh3)2RuL](ClO4) (L = C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CFc, [1](ClO4); C
CFc, [1](ClO4); C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C6H4–C
C–C6H4–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CFc), [2](ClO4); C
CFc), [2](ClO4); C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C6H4–C6H4–C
C–C6H4–C6H4–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CFc, [3](ClO4)) were synthesized by the reactions of [(tppz)(PPh3)2RuCl](ClO4) (tppz = 2,3,5,6-tetrakis(2-pyridyl)pyrazine) with ferrocence-acetylide ligands and characterized by ESI-MS, and 1H and 31P NMR spectroscopies. The structure of [1](PF6) was determined by X-ray crystallography. The electrochemical studies show that compounds [1](ClO4)–[3](ClO4) possess two widely separated anodic peaks, ascribable to one-electron oxidation of Fc and RuII, respectively. This assignment is further corroborated by the results of UV-vis-NIR, XPS, and theoretical calculation studies. Compound [1](ClO4) exhibits significant Ru⋯Fe metal–metal interactions across the Ru–C
CFc, [3](ClO4)) were synthesized by the reactions of [(tppz)(PPh3)2RuCl](ClO4) (tppz = 2,3,5,6-tetrakis(2-pyridyl)pyrazine) with ferrocence-acetylide ligands and characterized by ESI-MS, and 1H and 31P NMR spectroscopies. The structure of [1](PF6) was determined by X-ray crystallography. The electrochemical studies show that compounds [1](ClO4)–[3](ClO4) possess two widely separated anodic peaks, ascribable to one-electron oxidation of Fc and RuII, respectively. This assignment is further corroborated by the results of UV-vis-NIR, XPS, and theoretical calculation studies. Compound [1](ClO4) exhibits significant Ru⋯Fe metal–metal interactions across the Ru–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Fc backbone. As revealed by electrochemical, spectroscopic and theoretical computational studies, one-electron oxidized species [1a](ClO4)2 is between the electronically delocalized and valence-trapped state and shows a typical Robin–Day class II mixed-valence behavior.
C–Fc backbone. As revealed by electrochemical, spectroscopic and theoretical computational studies, one-electron oxidized species [1a](ClO4)2 is between the electronically delocalized and valence-trapped state and shows a typical Robin–Day class II mixed-valence behavior.

 Please wait while we load your content...
Please wait while we load your content...