MnBr2/18-crown-6 coordination complexes showing high room temperature luminescence and quantum yield†
Abstract
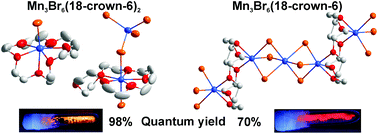
The reaction of manganese(II) bromide and the crown ether 18-crown-6 in the ionic liquid [(n-Bu)3MeN][N(Tf)2] under mild conditions (80–130 °C) resulted in the formation of three different coordination compounds: MnBr2(18-crown-6) (1), Mn3Br6(18-crown-6)2 (2) and Mn3Br6(18-crown-6) (3). In general, the local coordination and the crystal structure of all compounds are driven by the mismatch between the small radius of the Mn2+ cation (83 pm) and the ring opening of 18-crown-6 as a chelating ligand (about 300 pm). This improper situation leads to different types of coordination and bonding. MnBr2(18-crown-6) represents a molecular compound with Mn2+ coordinated by two bromine atoms and only five oxygen atoms of 18-crown-6. Mn3Br6(18-crown-6)2 falls into a [MnBr(18-crown-6)]+ cation – with Mn2+ coordinated by six oxygen atoms and Br – and a [MnBr(18-crown-6)MnBr4]− anion. In this anion, Mn2+ is coordinated by five oxygen atoms of the crown ether as well as by two bromine atoms, one of them bridging to an isolated (MnBr4) tetrahedron. Mn3Br6(18-crown-6), finally, forms an infinite, non-charged 1∞[Mn2(18-crown-6)(MnBr6)] chain. Herein, 18-crown-6 is exocyclically coordinated by two Mn2+ cations. All compounds show intense luminescence in the yellow to red spectral range and exhibit remarkable quantum yields of 70% (Mn3Br6(18-crown-6)) and 98% (Mn3Br6(18-crown-6)2). The excellent quantum yield of Mn3Br6(18-crown-6)2 and its differentiation from MnBr2(18-crown-6) and Mn3Br6(18-crown-6) can be directly correlated to the local coordination.

 Please wait while we load your content...
Please wait while we load your content...