Fluorinated triazapentadienyl ligand supported ethyl zinc(ii) complexes: reaction with dioxygen and catalytic applications in the Tishchenko reaction†
Abstract
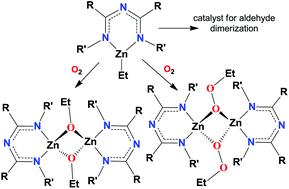
Ethyl zinc complexes [N{(C3F7)C(Dipp)N}2]ZnEt, [N{(C3F7)C(Cy)N}2]ZnEt, [N{(CF3)C(2,4,6-Br3C6H2)N}2]ZnEt and [N{(C3F7)C(2,6-Cl2C6H3)N}2]ZnEt have been synthesized from the corresponding 1,3,5-triazapentadiene and diethyl zinc. X-ray data show that [N{(C3F7)C(Dipp)N}2]ZnEt has a distorted trigonal planar geometry at the zinc center. The triazapentadienyl ligand binds to zinc in a κ2-mode. The zinc-ethyl bonds of [N{(C3F7)C(Dipp)N}2]ZnEt, [N{(C3F7)C(Cy)N}2]ZnEt, [N{(CF3)C(2,4,6-Br3C6H2)N}2]ZnEt and [N{(C3F7)C(2,6-Cl2C6H3)N}2]ZnEt readily undergo oxygen insertion upon exposure to dry air to produce the corresponding zinc-ethoxy or zinc-ethylperoxy compounds. The ethoxy zinc adducts {[N{(CF3)C(2,4,6-Br3C6H2)N}2]ZnOEt}2 and {[N{(C3F7)C(2,6-Cl2C6H3)N}2]ZnOEt}2 as well as the ethylperoxy zinc adduct {[N{(C3F7)C(Cy)N}2]ZnOOEt}2 have been isolated and fully characterized by several methods including X-ray crystallography. They feature dinuclear structures with four-coordinate zinc sites and bridging-ethoxy or -ethylperoxy groups. The ethyl zinc complexes catalyze the Tishchenko reaction of benzaldehyde under solventless conditions affording benzyl benzoate. The reaction of ethyl zinc complexes with dioxygen and their catalytic behaviour in the Tishchenko reaction are affected by the electronic and steric factors of the triazapentadienyl ligand. {[N{(C3F7)C(Cy)N}2]ZnOOEt}2 is an excellent reagent for the epoxidation of trans-chalcone.
- This article is part of the themed collection: Philip Power at 65: an icon of organometallic chemistry

 Please wait while we load your content...
Please wait while we load your content...