Ethylene oligomerization studies by nickel(ii) complexes chelated by (amino)pyridine ligands: experimental and density functional theory studies†
Abstract
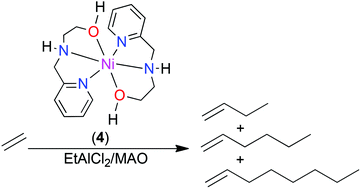
Reductions of imine compounds 2-methoxy-N-(1-(pyridin-2-yl)ethylidene)ethanamine (L1), 2-methoxy-N-((pyridin-2-yl)methylene)ethanamine (L2), N,N-diethyl-N-((pyridin-2-yl)methylene)ethane-1,2-diamine (L3) and 2-((pyridin-2-yl)methyleneamino)ethanol (L4) using NABH4 produced their corresponding amine analogues N-(2-methoxyethyl)-1-(pyridin-2-yl)ethanamine (L1a), 2-methoxy-N-((pyridin-2-yl)methyl)-ethanamine (L2a), N,N-diethyl-N-((pyridin-2-yl)methyl)ethane-1,2-diamine (L3a) and 2-((pyridin-2-yl)methylamino)ethanol (L4a) in good yields. Reactions of the (amino)pyridine ligands L1a–L4a with [NiBr2(DME)] afforded nickel(II) complexes, [NiBr2(L1a)2] (1), [NiBr2(L2a)2] (2), [NiBr2(L3a)2] (3) and [NiBr2(L4a)2] (4), respectively in quantitative yields. Molecular structures of complexes 2 and 4 confirmed the formation of the bis(chelated)nickel(II) complexes. Activation of complexes 1–4 with either EtAlCl2 or methylaluminoxane (MAO), produced active ethylene oligomerization catalysts to afford mostly ethylene dimers (C4), in addition to trimmers (C6) and tetramers (C8). Density functional theory studies provided valuable insight into the reactivity trends and influence of complex structure on the ethylene oligomerization reactions.

 Please wait while we load your content...
Please wait while we load your content...