Perovskite-structured AMnxB1−xO3 (A = Ca or Ba; B = Fe or Ni) redox catalysts for partial oxidation of methane
Abstract
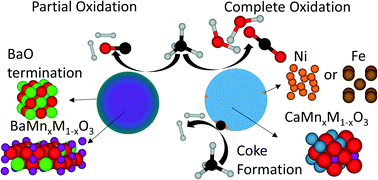
Methane is one of the simplest and most abundant organic compounds on earth. Methane reforming offers the versatility to produce value-added fuels and chemicals. Unlike typical reforming processes, which suffer from efficiency losses associated with endothermic reforming reactions and/or oxygen/steam generation, chemical looping reforming (CLR) utilizes the redox properties of an oxygen carrier, a.k.a. a redox catalyst, to partially oxidize methane into syngas with its lattice oxygen. The reduced redox catalyst is subsequently regenerated with air, providing in situ air separation with minimal energy penalty. The performance of the CLR process is highly dependent on the redox catalyst. In the current study, CLR performances and the underlying mechanisms for perovskite-structured redox catalysts with a general formula of AMnxB1−xO3 (A = Ca or Ba; B = Fe or Ni) are reported. CaMnO3 and BaMnO3 perovskites are worth investigating due to their desirable redox properties and relatively low cost. BaMnxB1−xO3 (B = Fe or Ni) based redox catalysts are shown to be more selective and coke-resistant to methane partial oxidation when compared to CaMnxB1−xO3 (B = Fe or Ni) based redox catalysts. While undoped BaMnO3 exhibited high selectivity towards syngas, addition of B-site dopants such as Ni or Fe leads to higher oxygen carrying capacity without significantly impacting the coke resistance of the redox catalysts. In contrast, nickel doped CaMnO3 is significantly more prone to coke formation. As a low cost and environmentally benign option, a BaMnxFe1−xO3 based redox catalyst was tested for CLR operations in a fluidized bed reactor. >95% syngas selectivity was observed with no signs of deactivation over 20 cycles.

 Please wait while we load your content...
Please wait while we load your content...