Carbon nanofiber-supported ReOx catalysts for the hydrodeoxygenation of lignin-derived compounds†
Abstract
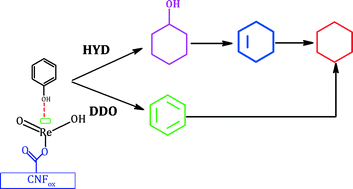
The effect of ReOx loading (2–13 wt%) and H2 pressure (0–5 MPa) for the hydrodeoxygenation of phenol has been studied for carbon nanofiber-supported ReOx catalysts in a batch reactor at 573 K. Characterization of the supports and catalysts has been obtained from N2 physisorption, TPD, FTIR, XRD, potentiometric titration, TPR and XPS measurements, which revealed the presence of a crystalline and surface ReOx phase whose particle size and surface coverage increased with loading. The reactivity of the catalysts was linked to the in situ partial reduction of ReO3 to form Re4+ and Re6–7+ sites, whose presence and relative amounts were determined by post-reaction XPS analysis. The reaction rate increased with ReOx loading up to 10 wt%, attributed to the increase in Re surface coverage; a decrease in reaction rate at higher loading was ascribed to the formation of aggregates. The study revealed a strong affinity for direct cleavage of the C–O bond to form benzene. The similar relative abundance of the Re species is responsible for the similar trend in product distribution of the catalysts. The dependence of activity and product distribution with respect to H2 pressure has been related to kinetics and thermodynamics. The reactivity of the best catalyst for the HDO of guaiacol (2-methoxyphenol), anisole (methoxybenzene), phenol and o-cresol (2-methylphenol) further demonstrated the catalyst's preference for C–O bond scission.

 Please wait while we load your content...
Please wait while we load your content...