Electric field effects on the electronic properties of the silicene–amine interface
Abstract
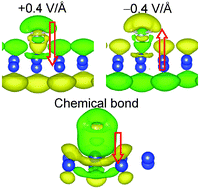
We performed first-principles studies of electric field (EF) effects on the electronic properties of silicene–amine (NH3 and NH2CH3) hetero-interface systems focusing on the electronic interactions at the interface. The band gaps of the systems increase with a positive applied EF but decrease with a negative EF; that is, the band gaps monotonically vary on changing the applied EF from negative to positive. The phenomenon of band gap variation with the sign of the applied EF is a characteristic feature of hetero-interface systems. We revealed the mechanism of the electronic structure change in silicene–amine due to an applied EF by visualizing the electron density change. It is shown that the electronic polarizations in both the Si–N chemical bond region and the silicene-layer region determine the characteristic band gap variation. Furthermore, the tunable energy range of the band gap of the silicene–amine is considerably higher than the range of a silicene monolayer; thus, the idea of controlling the band gaps of hetero-interface systems in combination with application of an EF bias is suitable for designing various devices that are difficult to fabricate with homogeneous two-dimensional materials such as silicene and graphene.

 Please wait while we load your content...
Please wait while we load your content...