When a nanoparticle meets a superhalogen: a case study with C60 fullerene†
Abstract
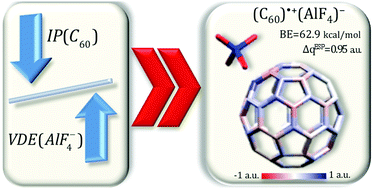
The ability of a selected nanoparticle to form stable systems with superhalogens (i.e. AlF4, AlCl4, MgF3, MgCl3, LiF2, LiCl2, and LiI2) is examined on the basis of theoretical considerations supported by ab initio calculations. It is demonstrated that the C60 fullerene molecule should form stable and strongly bound (C60)˙+(superhalogen)− radical cation salts when combined with an appropriately chosen superhalogen radical (acting as an oxidizing agent). The conclusion is supported by providing: (i) the structural deformation of superhalogens and C60 nanoparticles upon ionization, (ii) predicted charge flow between the fullerene and each superhalogen (which allows estimating the amount of electron density withdrawn from the C60 molecule during the ionization process), (iii) the localization of the spin density distribution, and (iv) the interaction energies for the compounds obtained both at the B3LYP/6-31+G(d) level and at the B3LYP-D3/6-31+G(d) level. Solvent effects have been considered in the present study by means of the polarizable continuum model. It is found that the stability of C60/superhalogen species can be improved in solvents. We believe that the results provided in this contribution may likely be of prospective relevance in the future studies on the issue of binding and removal of this potentially risky nanoparticle.

 Please wait while we load your content...
Please wait while we load your content...