Assessing the formation of weak sodium complexes with negatively charged ligands
Abstract
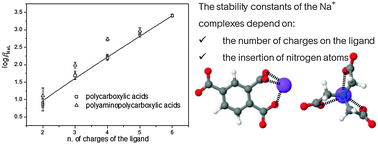
The stability of sodium complexes with poly-carboxylic and polyamino-carboxylic acids is investigated with ion-selective electrode-Na+ potentiometry, working at strictly constant ionic strength. It is observed that the formation constants of the Na+ complexes with monoligand stoichiometry (ML) increase with the number of charges on the ligand. For example, in poly-carboxylic acids this dependency is linear and is well captured by an experimental equation. A different behaviour is observed for the poly-amino carboxylic acids, which show higher complexation capabilities reaching a plateau of the binding energy past a specific ligand size. The experimental results are discussed qualitatively using ab initio calculations based on DFT B3LYP, and the principal electronic characteristics of the ligands under investigation are identified. As a result of the flexibility imparted by the long chains of polyamino-carboxylic ligands, both experimental and theoretical models demonstrate that nitrogen atoms in proximity of Na+ ions can participate in the metal coordination, thus providing further stabilization for the complexes. Moreover, by increasing the ligand size the stabilization gained in terms of ΔG reached a plateau for EDTA, in agreement with experimental observations.


 Please wait while we load your content...
Please wait while we load your content...