Isodesmic reaction for accurate theoretical pKa calculations of amino acids and peptides†
Abstract
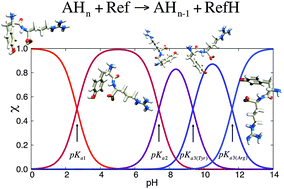
Theoretical and quantitative prediction of pKa values at low computational cost is a current challenge in computational chemistry. We report that the isodesmic reaction scheme provides semi-quantitative predictions (i.e. mean absolute errors of 0.5–1.0 pKa unit) for the pKa1 (α-carboxyl), pKa2 (α-amino) and pKa3 (sidechain groups) of a broad set of amino acids and peptides. This method fills the gaps of thermodynamic cycles for the computational pKa calculation of molecules that are unstable in the gas phase or undergo proton transfer reactions or large conformational changes from solution to the gas phase. We also report the key criteria to choose a reference species to make accurate predictions. This method is computationally inexpensive and makes use of standard density functional theory (DFT) and continuum solvent models. It is also conceptually simple and easy to use for researchers not specialized in theoretical chemistry methods.

 Please wait while we load your content...
Please wait while we load your content...