Hydrogen adsorption in azolium and metalated N-heterocyclic carbene containing MOFs†
Abstract
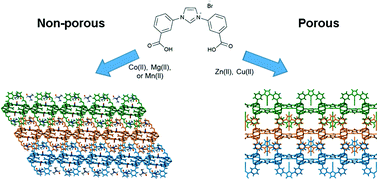
Azolium and metalated N-heterocyclic carbene (NHC) functionalised metal–organic frameworks (MOFs) have been investigated as adsorbents and heterogenous catalysts. Here we describe the structures of two sets of 1D polymeric structures, {[M2(μ2-HCOO)(HL)2](NO3)·xDMF}n (M = Zn, x = 1, 1; Cu, x = 1.75, 2) and {[M3(HL)4(H2O)4](NO3)2·xDMF}n (M = Co, x = 2.75, 3; Mg, x = 0, 4; Mn, x = 1.5, 5), prepared from reactions of 1,3-bis(3-carboxyphenyl)-1H-imidazol-3-ium bromide (H3LBr) with M(NO3)2·xH2O (M = Zn, Cu, Co and Mg) and MnCl2·4H2O. These pack as porous and non-porous 3D materials, respectively. In the case of the known Zn(II) material 1, which we reveal to be porous, we also report metalation of the NHC precursor concomitant with framework synthesis to give {[Zn2(μ2-HCOO)(HL)1.6(L–Cu–Br)0.4](NO3)0.6·0.75DMF}n (1-Cu). Compounds 1, 2, and 1-Cu show H2 adsorption enthalpies of −9.9, −9.1, and −9.7 kJ mol−1 respectively, and allow the effect of micropores decorated with charged imidazolium moieties, or NHC–CuBr entities, on H2 adsorption to be probed.


 Please wait while we load your content...
Please wait while we load your content...