Synthesis and photocatalytic activity of hexagonal phase NaYF4:Ho3+@TiO2 core–shell microcrystals
Abstract
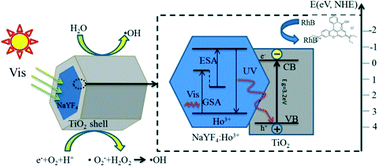
A β-NaYF4:Ho3+@TiO2 core–shell microcrystal photocatalyst was synthesized using a hydrothermal method followed by hydrolysis of tetra-n-butyl titanate (TBOT), with polyvinylpyrrolidone K-30 (PVP) as the coupling agent. X-ray diffraction, scanning electron microscopy, energy-dispersive X-ray spectroscopy, transmission electron microscopy, X-ray photoelectron spectroscopy, fluorescence and Raman spectrometry, ultraviolet-visible diffuse reflectance spectroscopy, and electron spin resonance were used to characterize the photocatalyst. It was found that the core's hexagonal phase NaYF4:Ho3+ microcrystals were evenly coated by a TiO2 shell and that the average β-NaYF4:Ho3+ microcrystal length was roughly 9 μm with a diameter of about 3.8 μm. After doping which caused a slight increase, the average thickness of the TiO2 shells was about 50 nm. Because Ho3+-single-doped β-NaYF4:Ho3+@TiO2 displays strong visible absorption peaks at 450, 537, and 642 nm, we chose 450, 532, and 633 nm to be the excitation wavelengths. We found ultraviolet emission bands at 290 nm (5D4 → 5I8) and 389 nm (3K7/5G4 → 5I8). Also, the energy transfer from β-NaYF4:Ho3+ to anatase TiO2 was confirmed. Rhodamine B (RhB) was used as a model pollutant to investigate the photocatalytic activity of β-NaYF4:Ho3+@TiO2 microcrystals under Xe lamp (500 W) irradiation. This investigation showed an advanced visible-light-driven catalyst, with about 67% of the RhB decomposed after 10 h. Compared with the blank experiment, the efficiency was obviously improved. Recycling experiments showed that the β-NaYF4:Ho3+@TiO2 composite still presented significant photocatalytic activity after four successive cycles. Finally, we investigated the β-NaYF4:Ho3+ upconversion (UC) mechanism and the β-NaYF4:Ho3+@TiO2 core–shell microcrystal visible-light-responsive photocatalytic mechanism. Understanding the visible-light-responsive photocatalytic mechanism will help to improve the structural design and functionality of this new type of catalytic material.

 Please wait while we load your content...
Please wait while we load your content...