Halogen bonding in a multi-connected 1,2,2-triiodo-alkene involving geminal and/or vicinal iodines: a crystallographic and DFT study†
Abstract
Four halogen-bonded arrangements of (1,2,2-triiodovinyl)benzene involving geminal and/or vicinal iodine atoms were studied both by X-ray diffraction and density functional theory (DFT). Crystallographic data show weaker XB-type interactions for the iodine atom geminal to the phenyl ring, which is corroborated by DFT-optimized structures of 1 : 1, 1 : 2 and 1 : 3 (1,2,2-triiodovinyl)benzene/pyridine complexes. In addition, a theoretical model reflects an interplay existing between these conjugated and multi-connected sites, highlighting the weakening of the halogen bonds when the number of partners is increased.
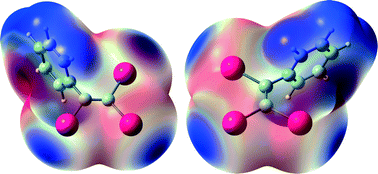

 Please wait while we load your content...
Please wait while we load your content...