Diastereoselective construction of cyclopent-2-enone-4-ols from aldehydes and 1,2-allenones catalyzed by N-heterocyclic carbene†
Abstract
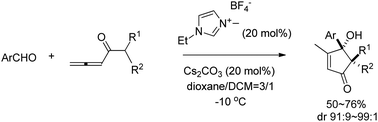
Cyclopent-2-enone-4-ols have been prepared highly diastereoselectively from readily available aromatic aldehydes and 1,2-allenones catalyzed by N-heterocyclic carbene. Mechanistic studies showed that there are four possible pathways.

 Please wait while we load your content...
Please wait while we load your content...