Low electric field strength self-organization of anodic TiO2 nanotubes in diethylene glycol electrolyte
Abstract
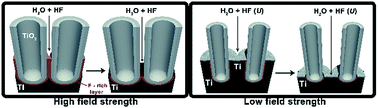
Self-organization of TiO2 nanotubes with large interconnecting space in-between the tubes has been demonstrated by the anodization of titanium in diethylene glycol/HF electrolyte containing a desired amount of water. The unique morphological features are a consequence of low electric field strength conditions, leading to the growth of tubes on a low population of nucleation sites. The proposed growth model assumes the presence of a metallic titanium in-between the tube cells that are oxidized/etched, resulting in the generation of an inhomogeneous oxide at the bottom of the nanostructure. The presented work contributes to the research field by the following aspects: (i) the low field strength conditions have been demonstrated to have an impact on the tube spacing, (ii) the water content in the electrolyte allows the precise control of the interconnecting space in-between the tubes, (iii) the tubes separation is controlled by the presence of Ti in-between the tube cells.

 Please wait while we load your content...
Please wait while we load your content...