Ammonolytical conversion of microcrystalline gallium antimonide GaSb to nanocrystalline gallium nitride GaN: thermodynamics vs. topochemistry
Abstract
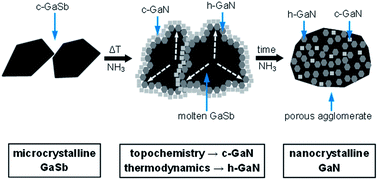
Reaction of microcrystalline powders of readily available gallium antimonide GaSb with ammonia gas at elevated temperatures afforded in one step high yields of nanocrystalline powders of the semiconductor, gallium nitride GaN. In particular, temperatures of 900–1000 °C and suitable reaction times of 36–170 hours resulted in complete nitridation in the system. GaN was prepared as a mixture of the major stable hexagonal and the minor metastable cubic polytypes. Formation of the cubic GaN was consistent with topochemistry playing a meaningful role in the ammonolysis of the cubic GaSb substrate. Specific experimental conditions, including variations in the reaction temperature/time and manual grinding or high energy ball milling of the substrate, had a significant impact on the final GaN polytype make-up and average crystallite size, the latter ranging from a few to a few tens of nanometers. Under the applied conditions, all by-products were conveniently removed from the reaction mixture as volatile species, thus affording chemically pure GaN nanopowders of very good quality.

 Please wait while we load your content...
Please wait while we load your content...