Ionic liquid entrapment by an electrospun polymer nanofiber matrix as a high conductivity polymer electrolyte
Abstract
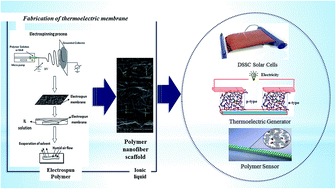
Through external doping, novel conductive polymer nanofibers were successfully fabricated using ionic liquids. In this study, a polymer blend of polyvinyl alcohol (PVA) and chitosan (CS) in a 4 : 1 weight ratio was fabricated in the form of nanofibers through electrospinning and used as a scaffold membrane to capture room-temperature ionic liquids (RTILs), such as 1-ethyl-3-methylimidazolium chloride (EMIMCl) and 1-butyl-3-methylimidazolium bromide (BMIMBr). Morphological analysis using scanning electron microscopy (SEM) showed that the scaffold structure of the electrospun membrane facilitated sufficient trapping of RTILs. This membrane demonstrated significantly increased conductivity from 6 × 10−6 S cm−1 to 0.10 S cm−1, interestingly surpassing the value of pure ionic liquids, where the polymer chain breathing model has been suggested as a hypothesis to explain this phenomena. The dominance of ions as charge carriers was explained using an ionic transference number measurement. The interaction between the polymer nanofiber matrix and an ionic liquid has been explained using Fourier-transform infrared spectroscopy (FTIR), where the ionic liquid was found to be physically dispersed in the polymer nanofiber matrix. These materials have also shown some thermoelectric (TE) activity, by demonstrating Seebeck coefficients up to 17.92 μV K−1. The existence of freely movable ions in this type of membrane shows their applications as energy storage/conversion devices such as organic thermoelectrics (TEs), sensors, and dye-sensitised solar cells.

 Please wait while we load your content...
Please wait while we load your content...