Asymmetric synthesis of (αS)-polyfluoroalkylated N-Boc-prolinols by the diethyl zinc-induced asymmetric Meerwein–Ponndorf–Verley reduction of perfluoroalkyl N-Boc-pyrrolidyl ketones†
Abstract
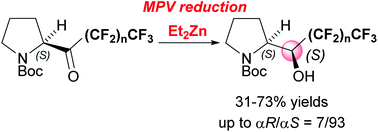
The reduction of perfluoroalkyl N-Boc-pyrrolidyl ketones with diethyl zinc was investigated. As a result, asymmetric Meerwein–Ponndorf–Verley reduction of perfluoroalkyl N-Boc-pyrrolidyl ketones proceeded smoothly with the use of 5 equiv. of diethyl zinc as a reducing agent in hexane at room temperature to give (αS)-polyfluoroalkylated N-Boc-prolinols in good yields (31–73%) with high diastereomer ratios (up to αR/αS = 7/93). The absolute configuration at the α-position of the major diastereomer is opposite to that obtained by the reduction of N-Boc-pyrrolidyl ketone with NaBH4 in ethanol. Furthermore, we also achieved the tandem perfluorobutylation-MPV reduction of N-Boc-proline ethyl ester to give (αS)-perfluorobutylated N-Boc-prolinol as a sole diastereomer in 45% yield.
- This article is part of the themed collection: Celebrating the 80th Birthday of Professor Ei-ichi Negishi

 Please wait while we load your content...
Please wait while we load your content...