Solution thermodynamics, computational and relaxometric studies of ditopic DO3A-based Mn(ii) complexes†
Abstract
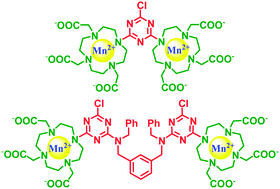
Mn(II)-complexes are experiencing renewed interest as MRI contrast agents due to safety concerns related to the use of Gd(III)-chelates. While the preparation of oligomeric and polymeric Gd(III)-chelates is widely employed to multiply their efficiency, scarce examples of this strategy are reported for the corresponding Mn(II)-complexes. Two novel ditopic DO3A-based ligands were designed and synthesized and their Mn(II)-complexes evaluated in order to shed more light on the correlation of the paramagnetic properties of the resultant dinuclear complexes with their structural features. The stability of these complexes was assessed by potentiometric titration, while modelling was adopted to gain the structural features. A detailed relaxometric characterization provided a defined picture of the paramagnetic properties of these dinuclear chelates, paving the way for the design of Mn(II)-based multimeric MRI contrast agents.

 Please wait while we load your content...
Please wait while we load your content...