In silico spatial simulations reveal that MCC formation and excess BubR1 are required for tight inhibition of the anaphase-promoting complex†
Abstract
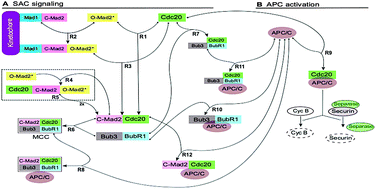
In response to the activation of the mitotic spindle assembly checkpoint (SAC), distinct inhibitory pathways control the activity of the anaphase-promoting complex (APC/C). It remains unclear whether the different regulatory mechanisms function in separate pathways or as part of an integrated signalling system. Here, five variant models of APC/C regulation were constructed and analysed. The simulations showed that all variant models were able to reproduce the wild type behaviour of the APC. However, only one model, which included both the mitotic checkpoint complex (MCC) as well as BubR1 as direct inhibitors of the APC/C, was able to reproduce both wild and mutant type behaviour of APC/C regulation. Interestingly, in this model, the MCC as well as the BubR1 binding rate to the APC/C was comparable to the known Cdc20–Mad2 binding rate and could not be made higher. Mad2 active transport towards the spindle mid-zone accelerated the inhibition speed of the APC/C but not its concentration level. The presented study highlights the principle that a systems biology approach is critical for the SAC mechanism and could also be used for predicting hypotheses to design future experiments. The presented work has successfully distinguished between five potent inhibitors of the APC/C using a systems biology approach. Here, the favoured model contains both BubR1 and MCC as direct inhibitors of the APC/C.

 Please wait while we load your content...
Please wait while we load your content...